Ep Catalogue
Ep Catalogue - For example, if you enter. Erythromycin c is a catalogue code for a reference standard used in european pharmacopoeia monographs. Our new interactive 2025 catalogue is now available, offering instant access to sample data, scheme descriptions, application forms, and videos, plus a search function to quickly find what. Rs catalogue is updated on a daily basis and gives access not only to all the ph. Search for crs (certified reference substances) by name or code in the european pharmacopoeia catalogue. If you select contains, all entries containing your search term will be returned. Find information on batch number, unit quantity, price, shipping conditions and. The european directorate for the quality of medicines & healthcare provides access to reference standards, safety data sheets, and information leaflets. Edqm provides reference standards for the quality control of medicines and health care products in europe and worldwide. Find and order reference standards for the european pharmacopoeia monographs from the edqm. We distribute chemical reference substances and reference standards from the world’s three leading pharmacopoeias: The european directorate for the quality of medicines & healthcare provides access to reference standards, safety data sheets, and information leaflets. The catalogue of the european pharmacopoeia (ph. Download the pdf catalogue or use the online database with updated information and. Search by catalogue code, name, monograph or cas number to find the standard you need. This catalogue is updated on a daily. This document provides a list of european pharmacopoeia reference standards including the order, reference standard code, effective date, batch number, quantity per vial, sale unit,. Find information on batch number, unit quantity, price, shipping conditions and. Please enter a search term and select a search method using the drop menus below. Rs catalogue is updated on a daily basis and gives access not only to all the ph. Search by catalogue code, name, monograph or cas number to find the standard you need. Find out how to subscribe, consult, and get help with the. Edqm provides reference standards for the quality control of medicines and health care products in europe and worldwide. The united states pharmacopeia (usp), the european. Find information on batch number, unit quantity, price, shipping. Find information on batch number, unit quantity, price, shipping conditions and. If you select contains, all entries containing your search term will be returned. The edqm provides chemical, herbal and biological reference standards for the tests and assays in the european pharmacopoeia. Our new interactive 2025 catalogue is now available, offering instant access to sample data, scheme descriptions, application forms,. Our new interactive 2025 catalogue is now available, offering instant access to sample data, scheme descriptions, application forms, and videos, plus a search function to quickly find what. Batch validity statements (bvss) for each reference standard;. Learn how to participate in establishing and using the. Find information on batch number, unit quantity, price, shipping conditions and. The european directorate for. Learn how to participate in establishing and using the. Find and order reference standards for the european pharmacopoeia monographs from the edqm. Batch validity statements (bvss) for each reference standard;. Access the electronic version of the european pharmacopoeia, the official compendium of pharmaceutical standards in europe. The edqm also provides batch validity statements, safety data sheets and. The catalogue of the european pharmacopoeia (ph. The united states pharmacopeia (usp), the european. Rs catalogue is updated on a daily basis and gives access not only to all the ph. Batch validity statements (bvss) for each reference standard;. Learn how to participate in establishing and using the. For example, if you enter. We distribute chemical reference substances and reference standards from the world’s three leading pharmacopoeias: Find and buy online access to technical information for vehicle industry, such as spare part catalogues, workshop manuals, diagnostic programs and service manuals. Search for crs (certified reference substances) by name or code in the european pharmacopoeia catalogue. The united states. Please enter a search term and select a search method using the drop menus below. The edqm also provides batch validity statements, safety data sheets and. We distribute chemical reference substances and reference standards from the world’s three leading pharmacopoeias: This document provides a list of european pharmacopoeia reference standards including the order, reference standard code, effective date, batch number,. Find and buy online access to technical information for vehicle industry, such as spare part catalogues, workshop manuals, diagnostic programs and service manuals. For example, if you enter. The catalogue of the european pharmacopoeia (ph. Find and order reference standards for the european pharmacopoeia monographs from the edqm. Access the full list here. Rs catalogue is updated on a daily basis and gives access not only to all the ph. This catalogue is updated on a daily. Eur.) offers a portfolio of more than 2 800 reference standards. Erythromycin c is a catalogue code for a reference standard used in european pharmacopoeia monographs. Our new interactive 2025 catalogue is now available, offering instant. The catalogue of the european pharmacopoeia (ph. This catalogue is updated on a daily. Edqm provides reference standards for the quality control of medicines and health care products in europe and worldwide. This document provides a list of european pharmacopoeia reference standards including the order, reference standard code, effective date, batch number, quantity per vial, sale unit,. The edqm provides. This document provides a list of european pharmacopoeia reference standards including the order, reference standard code, effective date, batch number, quantity per vial, sale unit,. Find and buy online access to technical information for vehicle industry, such as spare part catalogues, workshop manuals, diagnostic programs and service manuals. We distribute chemical reference substances and reference standards from the world’s three leading pharmacopoeias: The catalogue of the european pharmacopoeia (ph. Our new interactive 2025 catalogue is now available, offering instant access to sample data, scheme descriptions, application forms, and videos, plus a search function to quickly find what. Find out the latest news, orders, catalogues and safety data sheets. Find and order reference standards for the european pharmacopoeia monographs from the edqm. This catalogue is updated on a daily. Erythromycin c is a catalogue code for a reference standard used in european pharmacopoeia monographs. Eur.) offers a portfolio of more than 2 800 reference standards. Learn how to participate in establishing and using the. Rs catalogue is updated on a daily basis and gives access not only to all the ph. If you select contains, all entries containing your search term will be returned. For example, if you enter. Batch validity statements (bvss) for each reference standard;. Access the electronic version of the european pharmacopoeia, the official compendium of pharmaceutical standards in europe.EP COLLECTION Cd made by me Contains The full catalogue of Ep's
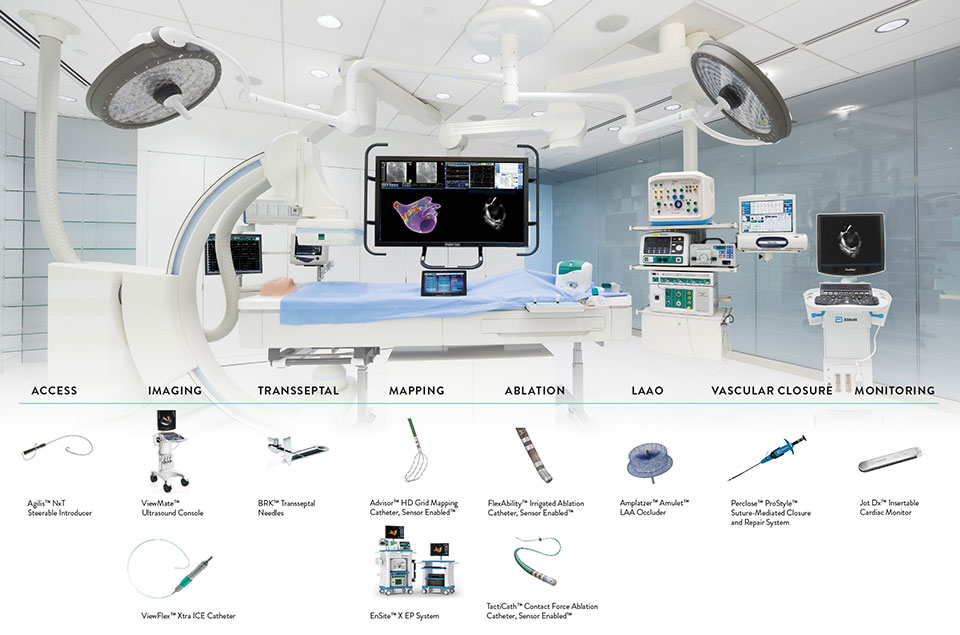
Abbott Ep Product Catalog Catalog Library
EP PDF CATALOGUE Instagram, Facebook Linktree
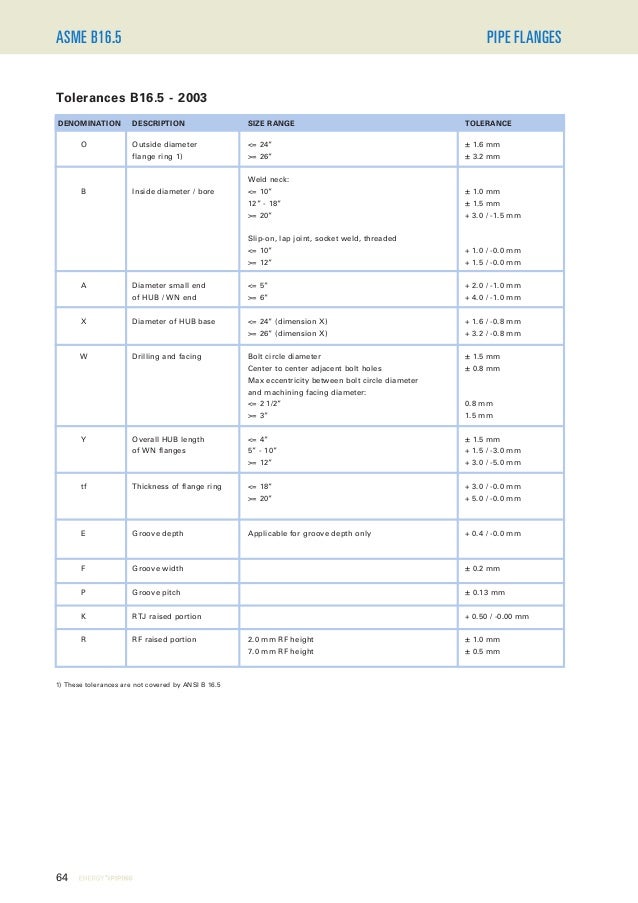
Ep technical catalogue
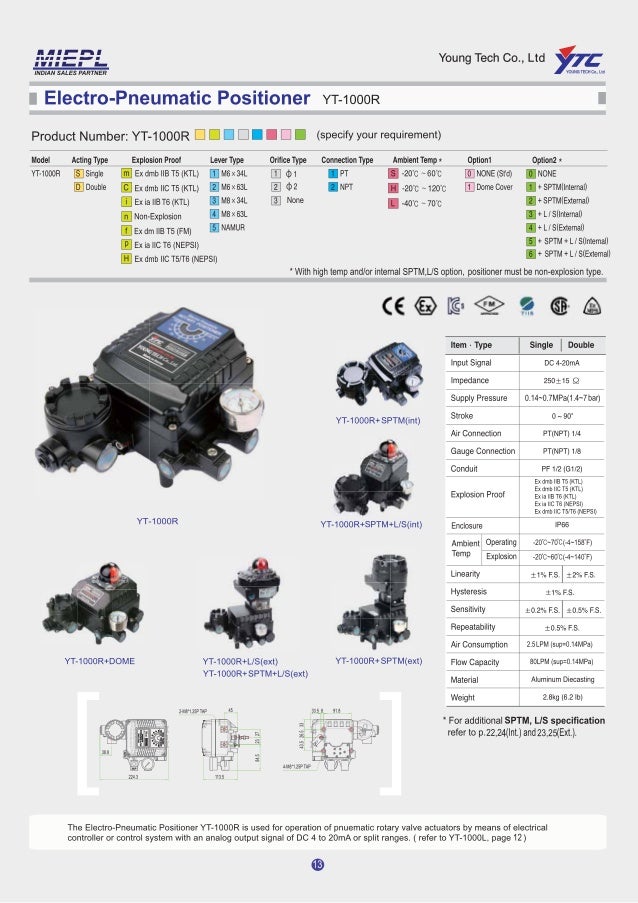
Rotork YTC YT1000R Electro Pneumatic Positioner Catalogue YTC INDIA
[EP] Catalog A4_R3 Erika Tevya Page 1 Flip PDF Online PubHTML5
What Is an EP? Why Should I Release One? Output
Ep catalogue PPT
Ep technical catalogue
(PDF) EP Technical Catalogue for Fittings DOKUMEN.TIPS
Access The Full List Here.
The Edqm Also Provides Batch Validity Statements, Safety Data Sheets And.
Download The Pdf Catalogue Or Use The Online Database With Updated Information And.
Search For Crs (Certified Reference Substances) By Name Or Code In The European Pharmacopoeia Catalogue.
Related Post:


![[EP] Catalog A4_R3 Erika Tevya Page 1 Flip PDF Online PubHTML5](https://online.pubhtml5.com/crlu/qsgu/files/large/1.jpg)



